Introductions
A quick Introduction to Soft Tissue Studies
Craniofacial Superimposition
Published on February 1, 2023
Written by María Alejandra Guativonza Higuera

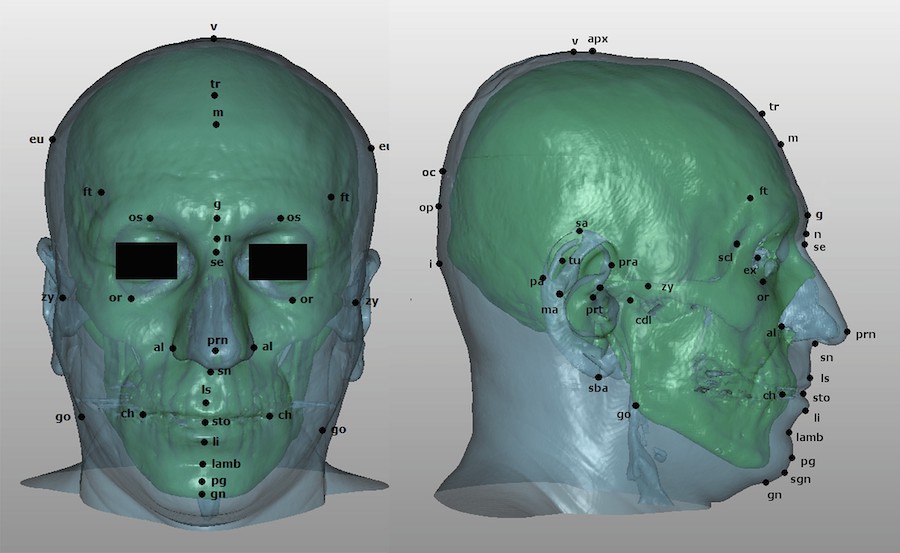
Quantifying the relationship between the skull and facial soft tissue is highly relevant for Craniofacial Identification techniques. Facial soft tissue thickness, measured as the distance between the surface of the skin and the surface of the underlying bone tissue at a specific pair of homologous craniometric and cephalometric landmarks, poses as an important set of criteria for the evaluation of anatomical consistency. Additionally, these measurements provide a basis for quantification and thus, repeatability of the results obtained in both Craniofacial Reconstruction (CFR) and Craniofacial Superimposition (CFS)
A number of studies have focused on measuring the facial soft tissue depth in a wide array of populations and using a variety of methods to obtain measurements. Without going into too much detail, dissection or needle puncture in cadavers are low-cost methods that yield immediate results and allow easy registration of soft tissue. However, there are some limitations since they provide an inaccurate representation of in vivo soft tissue due to cadaveric processes such as rigor mortis, bloating, loss of muscle mass, and embalming, as well as the cadaver being in the supine position.
Techniques such as palpation or direct measurement in live subjects are useful to establish relationships between facial surface and underlying bone tissue, but it does not allow direct observation of the tissues and there is a limited number of features that can be measured. Additionally, palpation and measuring objects might distort the soft tissue that is being measured if pressure is applied. This same drawback ensues with the use of ultrasound, due to applied pressure, while radiographs and computed tomographies (CT-CBCT) expose the subject to radiation. Conversely, MRI has none of these issues and an excellent display of three-dimensional soft tissue, although it shows an inferior display of bone, and is also acquired in the supine position.
Many authors [1]-[5] have warned about the wide variation in soft tissue depth measures acquired through different measurement techniques, irrespective of whether living persons or cadavers were considered, or the orthostatic or supine position of the subject. Other factors that influence the appearance of facial soft tissue are the differences in positional relationships associated with sex, age, or weight fluctuation.
Facial soft tissue plays a key role in CFS and CFR. Both techniques require the estimation of soft tissue thickness present at the locations of anthropometric landmarks. Currently, such estimation is performed by simply using average values obtained in population studies. This can limit the fidelity of the results, as average values are used to estimate very individual-specific pieces of information. Moreover, many populational studies have a relatively small sample size, thus the average values they provide are subject to noticeable sampling error. This is especially true when additional factors such as sex or age are also considered. For this reason, larger studies, even when not very specific to a population group, provide much more reliable average soft-tissue values and in turn lead to better results.
Stephan [6] has provided a metastudy that collects average values of multiple soft tissue thickness studies for both adults and sub-adults. This offers more accurate information that could be applied to different craniofacial techniques regardless of population. This study has proven useful when used in Skeleton-ID to apply the CFS technique in datasets from various populations. Soft tissue thickness average and standard deviation can be visualized in the form of cones once the overlay has taken place in-app.
As mentioned previously, the studies carried out have had issues regarding the distribution of their samples, such as age, sex, weight or population variation, sample size, inaccuracies associated with the different methods of acquisition, and frequently failed to address the direction of the measurement taken, the positional relationship between cranial and facial landmarks, the rate of muscle and fat within the soft tissue and other potentially relevant information.
Despite all this, the current circumstances are as favorable as ever, with CT and CBCT scanners widespread among dental clinics and hospitals. In particular, CBCT scans, which are taken in an orthostatic position (with the subject standing up and gravity affecting their facial features as it would in most photographs) are commonplace in dental clinics around the world.
At Panacea, we have automated multiple steps of the processing required to obtain soft tissue depth data from DICOM images, which means we can get results from larger samples much faster. If there is no study for the population of your interest, a quick fix would be to use Stephan’s metastudy, but if you want to take it into your own hands to create a population-specific study, you can contact us at pilotstudy@panacea-coop.com to collaborate with our research team.
Adult Soft Tissue Depth Data
| Population | Author(s) | Acquisition method |
|---|---|---|
| Australian | Simpson & Henneberg (2002), Domaracki & Stephan (2006), Stephan & Preisler (2018) | Needle puncture, ultrasound |
| Belgian | De Greef et al. (2009) | Ultrasound |
| Brazilian | Galdames et al. (2008), Tadeschi-Oliveira et al. (2009), Moritsugui et al. (2022) | Needle puncture, CBCT |
| Canadian | Vander Pluym et al. (2007), Peckmann et al. (2015) | MRI |
| Chilean | Galdames et al. (2008) | Ultrasound |
| Chinese | Birkner (1906), Stadtmuller (1923), Jia et al. (2016) | Ultrasound |
| Chinese-American | Chan et al. (2011) | Ultrasound |
| Egyptian | El-Mehallawi & Soliman (2001) | Ultrasound |
| French | Tilotta et al. (2009), Guyomarc’h et al. (2013) | CT |
| German | Welcker (1883), His (1895), Czekanowski (1907), Stadtmuller (1923), Berger (1965), Leopold (1968), Bankowski (1958), Helmer (1984) | Radiographs, needle puncture, ultrasound |
| Indian | Rhine (1983), Sahni et al. (2002), Meundi et al. (2019) | Needle puncture, MRI |
| Japanese | Suzuki (1948), Utsuno et al. (2014) | Radiographs |
| Korean | Hwang et al. (2012) | CBCT |
| Lebanese | Simpson, E., & Henneberg, M. (2002) | Needle puncture |
| Mediterranean | Ayoub et al. (2019) | Radiographs |
| Namibian | Von Eggeling (1904) | Needle puncture/dissection |
| Papuan | Fischer (1905) | Needle puncture |
| Portuguese | Codihna (2009) | Needle puncture |
| Slavic origins | Lebedinskaya & Veselovskaya (1986) | Ultrasound |
| South Africans | Phillips & Smuts (1996), Cavanagh & Steyn (2011), | CT |
| Sri Lankan | Sandamini et al. (2018) | MRI |
| Swiss | Kollman & Büchly (1899) | Needle puncture |
| Turkish | Kurkcuoglu et a.l (2011) | Radiographs |
| US black | Rhine & Campbell (1980), Manhein et al. (2000), Williamson & Nawrocki (2002) | Needle puncture, ultrasound |
| US hispanic | Manhein et al. (2000) | Ultrasound |
| US white | Weinig (1958), Hodson et al. (1985), George (1987), Manhein et al. (2000) | Radiographs, Ultrasound |
| US | Parks et al. (2014) | CT |
| Zulu | Aulsebrook et al. (1996) | Radiographs |
Sub-adult Soft Tissue Depth Data
| Population | Authors | Acquisition method |
|---|---|---|
| Canadian | Peckman et al. (2013) | Ultrasound |
| European | Dumont (1986), Nanda & Meng (1990) | Radiographs |
| Italian | Gibelli et al. (2016) | Radiographs |
| Japanese | Utsuno et al. (2004) (2010) | Radiographs |
| South African | Briers et al. (2015), Briers & Steyn (2018) | Radiographs |
| UK white | Wilkinson (2002) | Ultrasound |
| US black | Manhein et al. (2000) | Ultrasound |
| Us hispanic | Manhein et al. (2000) | Ultrasound |
| US white | Manhein et al. (2000) | Ultrasound |
Written by:
María Alejandra Guativonza
María Alejandra Guativonza Higuera is a pre-doctoral researcher at Panacea Cooperative Research. She holds a degree in Anthropology from Universidad de Los Andes (Bogotá, Colombia) and a master’s degree in Physical and Forensic Anthropology from Universidad de Granada (Granada, Spain) and is pursuing a Ph.D. at this institution.

She has worked on projects with Chemonics International Sucursal Colombia and the Colombian Missing Persons Search Unit (UBPD) with the help of the National Forensic Institute. She is currently part of the research team at Panacea Coop, focusing on craniofacial identification methods.
References
[1] A. Kurkcuoglu, C. Pelin, B. Ozener, R. Zagyapan, Z. Sahinoglu, and A. C. Yazici, ‘Facial soft tissue thickness in individuals with different occlusion patterns in adult Turkish subjects’, Homo, vol. 62, no. 4, pp. 288–297, Aug. 2011, doi: 10.1016/j.jchb.2011.06.001.
[2] C. N. Stephan, ‘Anthropological facial ’reconstruction–recognizing the fallacies, “unembracing” the errors, and realizing method limits’, Sci Justice, vol. 43, no. 4, pp. 193–200, Oct. 2003, doi: 10.1016/s1355-0306(03)71776-6.
[3] I. C. Suazo Galdames, M. Cantín López, D. A. Zavando Matamala, F. J. Perez Rojas, and S. R. Torres Muñoz, ‘Comparisons in Soft-Tissue Thicknesses on the Human Face in Fresh and Embalmed Corpses Using Needle Puncture Method’, International Journal of Morphology, vol. 26, no. 1, pp. 165–169, Mar. 2008, doi: 10.4067/S0717-95022008000100027.
[4] C. N. Stephan, B. Meikle, N. Freudenstein, R. Taylor, and P. Claes, ‘Facial soft tissue thicknesses in craniofacial identification: Data collection protocols and associated measurement errors’, Forensic Science International, vol. 304, p. 109965, Nov. 2019, doi: 10.1016/j.forsciint.2019.109965.
[5] O. Bulut, C.-Y. Liu, F. Koca, and C. Wilkinson, ‘Comparison of three-dimensional facial morphology between upright and supine positions employing three-dimensional scanner from live subjects’, Legal Medicine, vol. 27, Jun. 2017, doi: 10.1016/j.legalmed.2017.06.002.
[6] C. N. Stephan, ‘2018 tallied facial soft tissue thicknesses for adults and sub-adults’, Forensic Sci Int, vol. 280, pp. 113–123, Nov. 2017, doi: 10.1016/j.forsciint.2017.09.016.
[7] D. Sahni, null Sanjeev, G. Singh, I. Jit, and P. Singh, ‘Facial soft tissue thickness in northwest Indian adults’, Forensic Sci Int, vol. 176, no. 2–3, pp. 137–146, Apr. 2008, doi: 10.1016/j.forsciint.2007.07.012.
[8] E. Simpson and M. Henneberg, ‘Variation in soft-tissue thicknesses on the human face and their relation to craniometric dimensions’, Am J Phys Anthropol, vol. 118, no. 2, pp. 121–133, Jun. 2002, doi: 10.1002/ajpa.10073.
[9] H. Utsuno, T. Kageyama, K. Uchida, and K. Kibayashi, ‘Facial soft tissue thickness differences among three skeletal classes in Japanese population’, Forensic Sci Int, vol. 236, pp. 175–180, Mar. 2014, doi: 10.1016/j.forsciint.2013.12.040.
[10] F. Ayoub, M. Saadeh, G. Rouhana, and R. Haddad, ‘Midsagittal facial soft tissue thickness norms in an adult Mediterranean population’, Forensic Sci Int, vol. 294, p. 217.e1-217.e7, Jan. 2019, doi: 10.1016/j.forsciint.2018.10.021.
[11] P. Guyomarc’h, F. Santos, B. Dutailly, and H. Coqueugniot, ‘Facial soft tissue depths in French adults: variability, specificity and estimation’, Forensic Sci Int, vol. 231, no. 1–3, p. 411.e1–10, Sep. 2013, doi: 10.1016/j.forsciint.2013.04.007.
[12] H.-S. Hwang, M.-K. Park, W. J. Lee, J.-H. Cho, B.-K. Kim, and C. Wilkinson, ‘Facial Soft Tissue Thickness Database for Craniofacial Reconstruction in Korean Adults’, Journal of forensic sciences, vol. 57, May 2012, doi: 10.1111/j.1556-4029.2012.02192.x.
[13] M. Kaur, R. K. Garg, and S. Singla, ‘Analysis of facial soft tissue changes with aging and their effects on facial morphology: A forensic perspective’, Egyptian Journal of Forensic Sciences, vol. 5, no. 2, pp. 46–56, Jun. 2015, doi: 10.1016/j.ejfs.2014.07.006.
[14] M. Meundi and M. Chaya, ‘Facial soft tissue thickness in South Indian adults with varied occlusions – A cone beam computed tomography study’, vol. 31, no. 3, pp. 194–202, 2019.
[15] D. S. Moritsugui, F. V. G. Fugiwara, F. N. S. Vassallo, L. E. N. Mazzilli, T. L. Beaini, and R. F. H. Melani, ‘Facial soft tissue thickness in forensic facial reconstruction: Impact of regional differences in Brazil’, PLoS One, vol. 17, no. 7, p. e0270980, 2022, doi: 10.1371/journal.pone.0270980.
[16] T. L. Beaini, P. Miamoto, E. F. Duailibi-Neto, S. V. Tedeschi-Oliveira, I. Chilvarquer, and R. F. H. Melani, ‘Facial soft tissue depth measurements in cone-beam computed tomography: A study of a Brazilian sample’, Leg Med (Tokyo), vol. 50, p. 101866, May 2021, doi: 10.1016/j.legalmed.2021.101866.
[17] D. Cavanagh and M. Steyn, ‘Facial reconstruction: Soft tissue thickness values for South African black females’, Forensic Science International, vol. 206, no. 1–3, p. 215.e1-215.e7, Mar. 2011, doi: 10.1016/j.forsciint.2011.01.009.
[18] C. L. Parks, A. H. Richard, and K. L. Monson, ‘Preliminary assessment of facial soft tissue thickness utilizing three-dimensional computed tomography models of living individuals’, Forensic Sci Int, vol. 237, p. 146.e1-146.e10, Apr. 2014, doi: 10.1016/j.forsciint.2013.12.043.
[19] C. N. Stephan and R. Preisler, ‘In vivo facial soft tissue thicknesses of adult Australians’, Forensic Sci Int, vol. 282, p. 220.e1-220.e12, Jan. 2018, doi: 10.1016/j.forsciint.2017.11.014.
[20] W. N. J. Chan, G. A. Listi, and M. H. Manhein, ‘In vivo facial tissue depth study of Chinese-American adults in New York City’, J Forensic Sci, vol. 56, no. 2, pp. 350–358, Mar. 2011, doi: 10.1111/j.1556-4029.2010.01640.x.
[21] N. Briers and M. Steyn, ‘Re-assessment of South African juvenile facial soft tissue thickness data for craniofacial approximation: A comparative analysis using central tendency statistics’, Forensic Sci Int, vol. 291, p. 280.e1-280.e13, Oct. 2018, doi: 10.1016/j.forsciint.2018.08.008.
[22] D. Gibelli et al., ‘Variations of midfacial soft-tissue thickness in subjects aged between 6 and 18years for the reconstruction of the profile: A study on an Italian sample’, Leg Med (Tokyo), vol. 22, pp. 68–74, Sep. 2016, doi: 10.1016/j.legalmed.2016.08.005.
[23] T. R. Peckmann, M. H. Manhein, G. A. Listi, and M. Fournier, ‘In Vivo Facial Tissue Depth for Canadian Aboriginal Children: A Case Study from Nova Scotia, Canada’, Journal of Forensic Sciences, vol. 58, no. 6, pp. 1429–1438, 2013, doi: 10.1111/1556-4029.12211.
[24] T. R. Peckmann, M. Harris, M. Huculak, A. Pringle, and M. Fournier, ‘In vivo facial tissue depth for Canadian Mi’kmaq adults: A case study from Nova Scotia, Canada’, Journal of Forensic and Legal Medicine, vol. 29, pp. 43–53, Jan. 2015, doi: 10.1016/j.jflm.2014.12.004.
[25] H. Sandamini et al., ‘Facial soft tissue thickness trends for selected age groups of Sri Lankan adult population’, Forensic Sci Int, vol. 293, p. 102.e1-102.e11, Dec. 2018, doi: 10.1016/j.forsciint.2018.10.001.
[26] L. Jia, B. Qi, J. Yang, W. Zhang, Y. Lu, and H.-L. Zhang, ‘Ultrasonic measurement of facial tissue depth in a Northern Chinese Han population’, Forensic Science International, vol. 259, p. 247.e1-247.e6, Feb. 2016, doi: 10.1016/j.forsciint.2015.12.012.
[27] E. Weinig, ‘Die Nachweisbarkeit von Giften in exhumierten Leichen’, Dtsch. Z. ges. gerichtl. Med., vol. 47, no. 3, pp. 397–416, Sep. 1958, doi: 10.1007/BF00664116.
[28] H. Welcker, Schiller’s Schadel und Todtenmaske, nebst Mittheilungen uber Schadel und Todtenmaske Kant’s. Viehweg F and Son, Braunschweig, 1883.
[29] W. His, Johann Sebastian Bach’s Gebeine and Antiltz nebst Bemerkungen uber Dessen Bilder. Abhandlung durch Mathematic and Physic, 1895.
[30] J. Kollman and W. Büchly, ‘Die Persistenz der Rassen und die Reconstruction der Physiognomie prahistorischer Schadel.’, Archives fur Anthropologie, vol. 25, 1898.
[31] E. Fischer, ‘Anatomische Untersuchungen an den Kopfweichteilen zweier Papua.’, Corr BL Anthrop Ges Jhg, vol. 36, pp. 118–122, 1905.
[32] F. Birkner, ‘Beitrage zur Rassenanatomie der chinesen’, Korrespondenz Deutsches Gesicht Antropologie Ethnologie Urgeschte, vol. 35, no. 4, 1905.
[33] J. Czekanowski, ‘ntersuchungen uber das Verhaltnis der Kopfmafse zu den Schadelmafsen.’, Archiv fur Anthropologie, vol. 6, pp. 42–89, 1907.
[34] H. von Eggeling, ‘Anatomische untersuchungenan den Kopfen con cier Hereros, einem Herero- und einem Hottentottenkind’, in Forschungsreise im westrichen und zentraien Sudafrika, Schultze., Denkschriften, Jena, 1909.
[35] F. Stadtmuller, ‘Zur Beurteilung der Plastischen Rekonstruktions-Methods der Physiognomie auf dem Schadel’, Zeitschrift Fur Morphologie und antropologie, vol. 22, no. 35, 1925.
[36] K. Suzuki, ‘On the thickness of soft part of the Japanese face.’, J Anthropol Soc Nippon, vol. 60, no. 4, 1948.
[37] I. Bankowski, Die Bedeutung der Unterkieferform und—stellung für die photographische Schädelidentifizierung. 1958.
[38] D. Berger, Untersuchungen über die Weichteildickenmasse des Gesichts. 1965.
[39] J. Rhine, C. Moore, and J. Weston, Facial reproduction: tables of facial tissue thickness of American caucasoids in forensic anthropology, Albuquerque University of New Mexico. 1982.
[40] J. Rhine and H. Campbell, ‘Thickness of facial tissues in American blacks.’, Journal of Forensic Science, vol. 25, pp. 847–858, 1980.
[41] S. Rhine, Tissue thickness for South-western Indians. New Mexico, 1983.
[42] L. Farkas, Anthropometry of the head and face in medicine. Elsevier North Holland, New York, 1981.
[43] R. Helmer, ‘Schädelidentifizierung durch Elektronische Bildmischung.’, Kriminalstik-Verlag, 1984.
[44] G. Hodson, L. Lieberman, and P. Wright, ‘In vivo measurements of facial soft tissue thickness in American Caucasoid children.’, Journal of Forensic Science, vol. 30, pp. 1110–1112, 1985.
[45] E. Dumont, ‘Mid-facial tissue depths of white children: an aid in facial feature reconstruction.’, Journal of Forensic Science, vol. 31, pp. 1463–1469, 1986.
[46] R. M. George, ‘The Lateral Craniographic Method of Facial Reconstruction’, JFS, vol. 32, no. 5, pp. 1305–1330, Sep. 1987, doi: 10.1520/JFS11181J.
[47] R. Nanda and H. Meng, ‘Growth changes in the soft tissue facial profile.’, The Angle Orthodontist, vol. 60, pp. 177–190, 1990.
[48] W. Aulsebrook, P. Becker, and M. İşcan, ‘Facial soft-tissue thicknesses in the adult male Zulu.’, Forensic Science International, vol. 79, pp. 83–102.
[49] V. Phillips and N. Smuts, ‘Facial reconstruction: utilization of computerized tomography to measure facial tissue thickness in a mixed racial population.’, Forensic Science International, vol. 83, pp. 51–59, 1996.
[50] M. H. Manhein, G. A. Listi, R. E. Barsley, R. Musselman, N. E. Barrow, and D. H. Ubelaker, ‘In vivo facial tissue depth measurements for children and adults’, J Forensic Sci, vol. 45, no. 1, pp. 48–60, Jan. 2000.
[51] I. H. El-Mehallawi and E. M. Soliman, ‘Ultrasonic assessment of facial soft tissue thicknesses in adult Egyptians’, Forensic Sci Int, vol. 117, no. 1–2, pp. 99–107, Mar. 2001, doi: 10.1016/s0379-0738(00)00453-9.
[52] C. M. Wilkinson, ‘In vivo facial tissue depth measurements for white British children’, J Forensic Sci, vol. 47, no. 3, pp. 459–465, May 2002.
[53] M. A. Williamson, S. P. Nawrocki, and T. A. Rathbun, ‘Variation in midfacial tissue thickness of African-American children’, J Forensic Sci, vol. 47, no. 1, pp. 25–31, Jan. 2002.
[54] H. Utsuno, T. Kageyama, T. Deguchi, M. Yoshino, H. Miyazawa, and K. Inoue, ‘Facial soft tissue thickness in Japanese female children’, Forensic Sci Int, vol. 152, no. 2–3, pp. 101–107, Sep. 2005, doi: 10.1016/j.forsciint.2004.07.010.
[55] I. Leopold, ‘Trends in ocular therapy’, Am J Ophthalmol, vol. 65, pp. 297–317, 1968.

